How Many Electrons Are Unpaired in the Orbitals of Nitrogen
14 9 3 5 none of the above What is the electron configuration for Kr. Answer 3 unpaired electrons are present in the orbitals of nitrogen.

How Many Electrons Are Unpaired In N
We add the 12 valence electrons according to the Aufbau principle.

. In the lithium atom the two electrons in the 1s orbital are paired and the electron in the 2s orbital is. Why there are 3 unpaired electrons in nitrogen atom. Hence the number of unpaired electrons in nitrogen is 3.
Radicals are atoms or molecules with an unpaired electron. O 14 o 9 o 3 o 5 o none of the above. We review their content and use your feedback to keep the quality high.
How many unpaired electrons does Mn2 have. After arranging the electrons it is seen that the last shell of. Thus oxygen has two unpaired electrons and is paramagnetic.
Nitrogen is the seventh element with a total of 7 electrons. The paramagnetism is expressed in terms of magnetic moment. How many electrons are unpaired in the orbitals of nitrogen.
That is the number of electrons in nitrogen is 7. How many valence electrons does nitrogen ionN 3- have. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital.
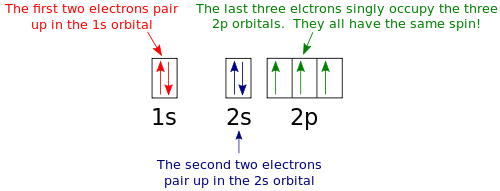
It has a total of 24 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Thus nitrogen contains 3 unpaired electrons one in each of the available p orbitals.
How many unpaired electrons are present in nitrogen. How many electrons are unpaired in the orbitals of nitrogen. Nitrogen is in group 5 and so has 5 outer electrons.
That means that it has only 3 electrons in the 2p orbitals. How many unpaired electrons are in lithium. And in N atoms four electrons are occupied by 1s and 2s orbitals and the remaining three are singly filled in the three orbitals present in the p subshell.
The atomic orbitals of the O atoms overlap to form the σ and π orbitals of the O2 molecule as shown in the diagram above. Which element is represented by the electron configuration 1s22s22p2. The only answer choice without unpaired electrons in its ground state is helium.
The atomic number for nitrogen is 7. Each of the 3 hydrogens is adding another electron to the nitrogens outer level making a total of 8 electrons in 4 pairs. The atomic number of nitrogen N is 7.
How many paired electrons does sodium have. From the Periodic Table or other description of the electron shells of nitrogen we see that its configuration is 1s2 2s2 2p3. Katejordan7655 katejordan7655 04222020 Chemistry High School answered How many electrons are unpaired in the orbitals of nitrogen.
Add your answer and earn points. Electronic configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbital. 2px2py2pz have 1 unpaired electron in each.
Thus the total no. Electronic configuration of N i 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 8. How many electrons are unpaired in the orbitals of nitrogen.
How many electrons are unpaired in the orbitals of nitrogen. How many outer shell electrons does sodium have. How many electrons are unpaired in the orbitals of carbon.
Hence there are 5 unpaired electrons in Mn2 How nitrogen has lone pairs. The magnetic moment of an atom. 3 When filling orbitals of equal energy electrons fill them singly first with parallel spins This is known as.
The remaining three electrons will. It can be easily concluded from the electronic configuration of N i. This can be told from the periodic table.
Neutral nitrogen must have both 7 electrons and 7 protons then. How many electrons are unpaired in the orbitals of nitrogen. Therefore a nitrogen atom will have two electrons in the first shell and five in the 2nd shell.
Therefore the order of the number of electrons in each shell of the nitrogen N atom is 2 5. In 1s 2s and 2p orbitals have two electrons so they are paired but in 3s orbital only one electron is present so Sodium has one unpaired electron. The p orbital of nitrogen ie.
If you were writing an electron. How many electrons are unpaired in the orbitals of nitrogen. An accepted abbreviation format is to write an electron configuration that includes a noble gas symbol in brackets.
How many unpaired electrons in N I atomic number 28. Get the answers you need now. These electrons are in the 2p orbital.
Here the electron configuration of nitrogen shows that three unpaired electrons exist. Recall from the chapter that a diamagnetic substance is identified by the lack of unpaired electrons in its shell. A 14 B 5 C 9 D 3.
1s2 2s2 2p6 3s2 3p6 4s2 3d2 4p6 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 1s2 2s2 2p6 3s2 3p4 4s2 3d10 4s2 4p6 1s2 2s2 2p6 3s2 3p2 4s2 3d10 4p6 none of the above. The first 7 orbitals are filled as 1s2 2s2 2p3 which shows us that nitrogen has 5 valence. 1 See answer Advertisement Advertisement katejordan7655 is waiting for your help.
Experts are tested by Chegg as specialists in their subject area. Of unpaired electrons in Nitrogen atom is 3. In this case the valency of the nitrogen atom is 3.
Chemistry questions and answers. How many electrons are unpaired in the orbitals of nitrogen. A 12 B 6 C 4 D 2.
Nitrogen element present in. The Substance which contain species with unpaired electrons in their orbitals behave as paramagnetic substances. How many unpaired electrons does nitrogen have in its ground state.
The number of unpaired electrons in N i atomic number 28 are The number of unpaired electrons in N i atomic number 28 are 2. A C B He C Be D O. Which molecule has unpaired electron.
What is the electron configuration for Kr.
High School Chemistry Orbital Configurations Wikibooks Open Books For An Open World

How Many Electrons Are Unpaired In The Orbitals Of Nitrogen Brainly Com
How Many Electrons Are Unpaired In The Valence Orbitals Of Nitrogen Quora
Comments
Post a Comment